Important: Retatrutide remains an investigational medicine. The UK Medicines and Healthcare products Regulatory Agency (MHRA) has not approved retatrutide for general prescribing as of February 19, 2026. See MHRA guidance | Learn more
Buy Retatrutide UK
Veyvora Retatrutide Pens
Veyvora sells retatrutide peptide pens in the UK. This page explains what retatrutide is, what clinical studies show, how dosing worked in trials, how the pen system operates, and what UK buyers should understand before purchasing.
Last updated: February 19, 2026
Key Takeaways
- •Retatrutide is a triple hormone receptor agonist that activates GLP-1, GIP, and glucagon receptors.
- •A Phase 2 trial published June 26, 2023 in The New England Journal of Medicine reported up to 24.2% mean weight reduction at 48 weeks at the highest studied dose.
- •Gastrointestinal side effects such as nausea and vomiting occurred more frequently at higher doses.
- •Retatrutide is not licensed in the UK for routine prescribing.
- •Veyvora supplies retatrutide pens with batch documentation and UK distribution.

Retatrutide Weight-Loss Journeys: UK Customer Progress Reports
The following accounts are voluntarily submitted by Veyvora customers in the United Kingdom. Individual outcomes differ based on baseline weight, adherence, diet, and exercise. These reports do not constitute medical advice or guarantee specific results.
14-22%
Self-Reported Reduction
12-24 wk
Typical Duration
Weekly
Dosing Schedule
CoA
Batch Verified
Steady Reduction Over Three Months
A 36-year-old male customer from Leeds reported consistent weight reduction across a 12-week period using Veyvora retatrutide pens at a titrated dose. He combined the protocol with a moderate calorie deficit and three weekly resistance sessions. His self-reported measurements showed progressive changes in waist circumference and overall bodyweight. He noted reduced appetite from week two onward and mild nausea during the first week of each dose increase.
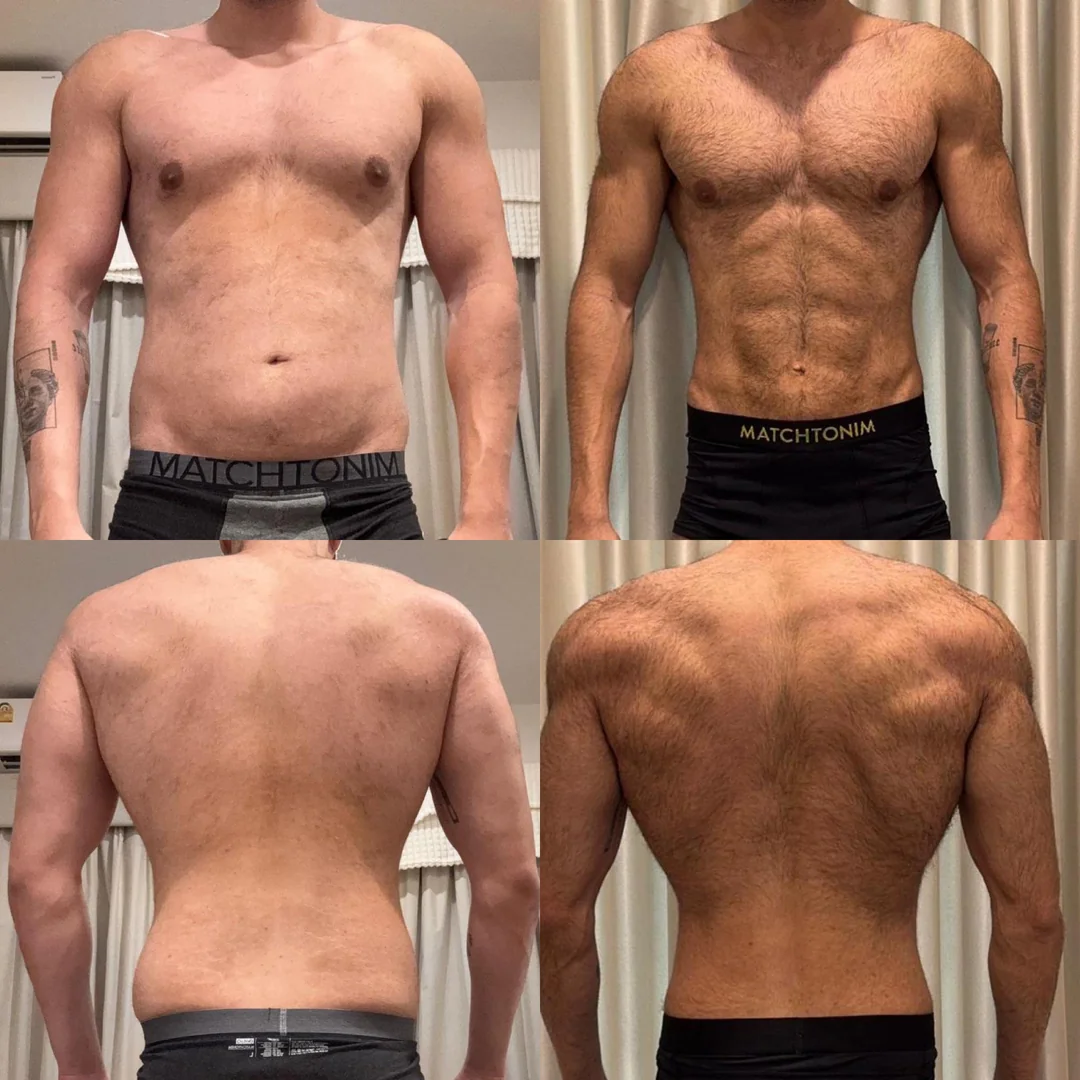
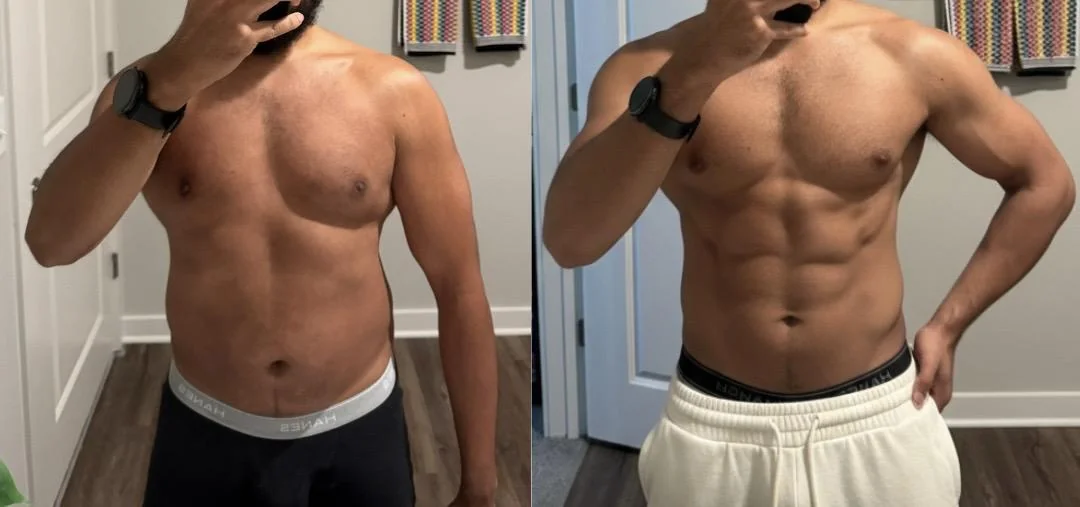
Extended Protocol with Body Recomposition
A 31-year-old male customer from Bristol documented his experience over a 24-week period. He followed a structured training programme alongside the retatrutide protocol, prioritising protein intake above 2 g/kg daily. His progress photographs, submitted voluntarily, show changes in visible muscle definition alongside fat reduction. He reported that appetite control was most noticeable in weeks 4 through 16, with mild gastrointestinal discomfort during the initial titration phase.
How do these reports compare to clinical trial data?
In the Phase 2 NEJM trial (Jastreboff et al., 2023), participants receiving 12 mg retatrutide achieved a mean 24.2% body weight reduction at 48 weeks under controlled conditions. Customer self-reports are not conducted under clinical supervision and should not be directly compared to peer-reviewed study outcomes. Variables including diet, exercise, starting BMI, and concurrent medications influence individual results.
Read the full clinical evidence behind retatrutide, including Phase 2 trial methodology and dose-response data, in our detailed research summary.
View Research and Regulatory InformationImportant: Testimonials and progress reports are submitted voluntarily by customers and reflect individual experiences. Veyvora does not independently verify weight or body composition claims. Retatrutide is an investigational peptide not approved by the MHRA for routine clinical use as of February 2026. These accounts do not constitute medical advice. Consult a qualified healthcare professional before using any peptide product.





Veyvora Retatrutide Pen
Retatrutide peptide pen with batch documentation and Certificate of Analysis. Triple-hormone receptor agonist targeting GLP-1, GIP, and glucagon receptors.
Free UK delivery | Batch documentation included | Pricing reflects manufacturing batch and strength
Veyvora sells retatrutide peptide pens in the UK. Retatrutide is an investigational triple agonist that activates GLP-1, GIP, and glucagon receptors. A Phase 2 trial published in The New England Journal of Medicine reported up to 24.2% mean weight reduction at 48 weeks. Each pen includes batch documentation.
Benefits of Veyvora Retatrutide Pens
Understand what retatrutide offers based on clinical trial data and what Veyvora provides with each order.
Activates GLP-1, GIP, and glucagon receptors simultaneously, distinguishing it from single and dual agonists.
Up to 24.2% mean weight reduction at 48 weeks at the highest studied dose in a randomized, placebo-controlled trial (NEJM 2023).
Veyvora retatrutide pens include batch documentation and Certificate of Analysis per batch.
The glucagon receptor activation increases energy expenditure by stimulating hepatic glucose output and fat oxidation.
Clinical evidence published in peer-reviewed journals including The New England Journal of Medicine.
Shipped within the UK with secure packaging and batch documentation included.
Ready to Buy Retatrutide in the UK?
Review product strength, confirm regulatory understanding, and complete checkout securely. Batch documentation and Certificate of Analysis included with every order.
What Do Clinical Studies Show?
A randomized, double-blind, placebo-controlled Phase 2 trial published in The New England Journal of Medicine on June 26, 2023 studied adults with obesity or overweight. At 48 weeks: 8 mg dose achieved 21.6% mean weight reduction, 12 mg dose achieved 24.2%, and placebo achieved 2.1%.
Source: Jastreboff AM et al. "Triple-Hormone-Receptor Agonist Retatrutide for Obesity." NEJM, 2023.
Landmark phase 2 trial demonstrating up to 24% body weight reduction with retatrutide over 48 weeks.
Read Full StudyComprehensive analysis of retatrutide's triple-hormone mechanism and its superior efficacy in weight management.
Read Full StudyCurrent phase 3 clinical trial evaluating long-term efficacy and safety of retatrutide in adults with obesity.
Read Full StudyOfficial Eli Lilly clinical development program for retatrutide, including multiple phase studies.
Read Full StudyDetailed pharmacological analysis of retatrutide's mechanism of action and metabolic effects.
Read Full StudyWhat are the risks observed?
Higher doses increased gastrointestinal adverse events. Participants reported nausea, vomiting, and diarrhea. Dose escalation reduced early intolerance. However, discontinuation rates increased at higher doses. Long-term cardiovascular outcome data are not yet published.
All studies referenced are conducted by independent research institutions. Retatrutide is currently in clinical development and available for research purposes only in the United Kingdom.
How to Use the Veyvora Retatrutide Pen: Step-by-Step Instructions
The Veyvora retatrutide pen delivers precision subcutaneous dosing via a pre-filled, multi-dose pen device. Retatrutide is administered once weekly in clinical studies. Follow these four steps for correct preparation, injection, and post-injection handling. Always consult a healthcare professional before use.

Keep the Veyvora retatrutide pen refrigerated between 2–8°C at all times when not in use. Do not freeze. Before each injection, remove the pen from the refrigerator and allow it to reach room temperature naturally for approximately 15–30 minutes. Injecting cold peptide solution can increase injection-site discomfort. Check the expiry date and batch number against the included Certificate of Analysis before first use.
Wash hands thoroughly. Remove the pen cap and attach a new, sterile pen needle by screwing it onto the pen tip until secure. Remove both the outer and inner needle caps. To prime, dial a small test dose as indicated in the pen instructions and press the injection button with the needle pointing upward until a drop of solution appears at the needle tip. Priming confirms the pen mechanism is functioning correctly and removes air from the cartridge. Never reuse needles between injections.

Dial the dose selector to the prescribed amount. In Phase 2 clinical trials published in The New England Journal of Medicine (Jastreboff et al., 2023), retatrutide was administered once weekly via subcutaneous injection. Recommended injection sites include the abdomen (at least 5 cm from the navel), the front of the thigh, or the back of the upper arm. Rotate injection sites each week to reduce the risk of lipodystrophy. Pinch the skin at the chosen site, insert the needle at a 90-degree angle, press the injection button fully, and hold for 5–10 seconds before withdrawing.

After injection, carefully replace the outer needle cap using a one-handed scoop technique to avoid needlestick injury. Unscrew and dispose of the used needle in an approved sharps container. Do not discard needles in household waste. Replace the pen cap and return the pen to refrigerated storage immediately. Record the injection date, dose, and site in a personal log to support consistent weekly administration and site rotation tracking. Each Veyvora pen includes batch documentation for your records.
Retatrutide Dosing in Clinical Trials
In Phase 2 trials, participants followed a gradual weekly dose escalation protocol. Starting doses were typically 1 mg weekly, increasing every four weeks until reaching a target of 8 mg or 12 mg. Gradual titration reduced gastrointestinal side effects. Rapid escalation increased nausea and discontinuation rates. This information is provided for educational context only.
Starting Dose (Trials)
1 mg
Once weekly, subcutaneous
Escalation Interval
4 weeks
Between dose increases
Target Doses (Trials)
8-12 mg
Highest studied doses
Quick-Reference Injection Checklist
Pre-Injection Preparation
- 1. Verify pen is within expiry date and batch matches Certificate of Analysis
- 2. Remove pen from refrigerator; allow 15-30 minutes to reach room temperature
- 3. Wash hands and attach a new sterile pen needle
- 4. Prime pen until a drop of solution appears at needle tip
Injection and Post-Injection
- 5. Dial prescribed dose and select a clean injection site (abdomen, thigh, or upper arm)
- 6. Pinch skin, inject at 90 degrees, hold button for 5-10 seconds
- 7. Dispose of needle in an approved sharps container
- 8. Return pen to 2-8 C storage and log date, dose, and injection site
Regulatory and Safety Notice
Retatrutide remains an investigational medicine. The UK Medicines and Healthcare products Regulatory Agency (MHRA) has not approved retatrutide for general prescribing as of February 19, 2026. Gastrointestinal adverse events including nausea, vomiting, and diarrhea were reported at higher doses in clinical trials. Long-term cardiovascular outcome data are not yet published. Always seek guidance from a qualified healthcare professional before using any peptide product.
Verified Veyvora Customer Reviews from Across the UK
Feedback submitted directly by UK customers about ordering, delivery, packaging, and product quality. Individual experiences may vary.
"Pen arrived next-day in insulated packaging. Certificate of Analysis matched the batch number printed on the label. No issues with the ordering process."
Edinburgh, UK
Ordered 1 pen, February 2026
"I contacted customer support with questions about storage requirements and they replied within a few hours with clear written guidance. Product was refrigerated on arrival."
Cardiff, UK
Repeat customer, 3 orders since November 2025
"Straightforward checkout. Tracking number was sent immediately. The pen mechanism functioned as described. I appreciated the batch documentation included in the box."
Glasgow, UK
Ordered 2 pens, January 2026
"Compared pricing with three other suppliers. Veyvora offered the most transparent breakdown and included third-party test results without having to request them separately."
Brighton, UK
First-time customer, December 2025
"Discreet delivery in a plain parcel. The pen was well-protected with foam inserts. Documentation inside covered storage, handling, and disposal instructions clearly."
Liverpool, UK
Ordered 1 pen, January 2026
"Placed the order on a Saturday and received it on Tuesday. Everything was correctly labelled and the cold chain appeared intact. Would use Veyvora again for future purchases."
Norwich, UK
Ordered 1 pen, February 2026
Order Retatrutide Pens from Veyvora
Every order includes batch documentation, Certificate of Analysis, and insulated delivery packaging. Select your preferred pen strength and complete checkout securely.
Reviews are submitted voluntarily and reflect individual customer experiences with ordering, delivery, and product quality. Veyvora does not edit or incentivise reviews.
What Is Retatrutide?
Retatrutide is an investigational triple agonist that targets three hormone receptors simultaneously:
GLP-1 Receptor
Glucagon-Like Peptide-1. Slows gastric emptying and reduces appetite.
GIP Receptor
Glucose-Dependent Insulinotropic Polypeptide. May improve insulin response.
Glucagon Receptor
Increases energy expenditure by stimulating hepatic glucose output and fat oxidation.
By activating all three pathways, retatrutide differs from:
- •Semaglutide (GLP-1 only; marketed as Ozempic and Wegovy)
- •Tirzepatide (dual GLP-1 and GIP agonist; marketed as Mounjaro)
This triple mechanism explains why researchers study retatrutide for obesity and metabolic disease.
Retatrutide vs Semaglutide
While Semaglutide (Ozempic, Wegovy) targets only the GLP-1 receptor, retatrutide activates GLP-1, GIP, and glucagon receptors. In Phase 2 trials, retatrutide achieved up to 24.2% mean weight reduction compared to semaglutide's approximately 15% in STEP trials. However, direct head-to-head comparisons are not yet published.
Read the full Retatrutide vs Semaglutide comparisonRetatrutide vs Tirzepatide: Triple vs Dual Action
Tirzepatide (Mounjaro) represents a significant advancement with its dual GLP-1/GIP mechanism, but retatrutide adds a third pathway: glucagon receptor activation. In trials, retatrutide achieved 24.2% vs tirzepatide's approximately 22.5% mean weight reduction. Researchers believe the glucagon component contributes to the higher weight reduction seen.
Read the full Retatrutide vs Tirzepatide comparison| Feature | Retatrutide | Tirzepatide | Semaglutide |
|---|---|---|---|
| GLP-1 agonist | Yes | Yes | Yes |
| GIP agonist | Yes | Yes | No |
| Glucagon agonist | Yes | No | No |
| Highest mean weight reduction (trial) | 24.2% | ~22.5% | ~15% |
Sources: NEJM Retatrutide 2023, NEJM Tirzepatide 2022 (SURMOUNT-1), NEJM Semaglutide 2021 (STEP 1)
How Does Retatrutide Work?
Retatrutide works by activating three hormone receptors that regulate appetite and metabolism.
GLP-1 Activation
Reduces hunger and slows gastric emptying.
GIP Activation
Enhances insulin secretion in response to meals.
Glucagon Activation
Increases energy expenditure.
This combined signaling reduces caloric intake and increases energy use. The glucagon component distinguishes retatrutide from dual agonists. Researchers believe this glucagon pathway contributes to the higher weight reduction seen in trials.
Retatrutide Dosing in Trials
Clinical trials used gradual weekly dose escalation. Participants typically began at a low dose (for example, 1 mg weekly). Researchers increased the dose every four weeks until reaching a target dose of 8 mg or 12 mg. See the full dosing guide.
Starting dose to allow body adaptation and reduce GI side effects
Gradual titration reduces gastrointestinal side effects
Target maintenance doses studied in trials
Important Dosing Notes
- Administered once weekly via subcutaneous injection
- Gradual titration reduced early intolerance
- Rapid escalation increased nausea and discontinuation
- Store pen at 2-8 C before use
- Allow pen to reach room temperature before injection
- Inject in abdomen, thigh, or upper arm
Benefits and Limitations
Reported Benefits in Trials
- Significant weight reduction compared to placebo
- Improvements in HbA1c (glycated hemoglobin) in participants with type 2 diabetes
- Dose-dependent appetite suppression
Limitations and Risks
- Gastrointestinal side effects increase with dose
- Long-term cardiovascular outcome data remain pending
- Retatrutide remains unapproved in the UK
Balanced evaluation matters. While weight reduction exceeded semaglutide trial averages (~15% in STEP trials), direct head-to-head comparisons are not yet published.
Where to Buy Retatrutide in the UK
Retatrutide remains investigational in the UK. Buyers should understand current regulatory status before purchase.
Batch-Tested Pens
Each batch independently tested for quality
UK Distribution
Shipped within the UK with secure packaging
Certificate of Analysis
Certificate of Analysis included per batch
Secure Checkout
Safe and secure payment processing
Pricing reflects manufacturing batch and strength and updates periodically. Check the product section for current pricing as of February 19, 2026.
Frequently Asked Questions
Is retatrutide the same as Ozempic?
No. Ozempic contains semaglutide, a GLP-1 receptor agonist only. Retatrutide activates GLP-1, GIP, and glucagon receptors.
Is retatrutide approved in the UK?
No. As of February 19, 2026, MHRA has not approved retatrutide for routine prescribing.
Why is retatrutide attracting attention?
Retatrutide produced higher mean weight reduction in Phase 2 trials compared to earlier GLP-1 agents. However, larger and longer studies must confirm durability and safety.
Final Considerations Before Buying
Retatrutide shows strong Phase 2 efficacy. However, it remains investigational and carries gastrointestinal side effects. Regulatory approval is pending. Buyers should understand both benefits and risks before purchasing.
Review product strength, confirm regulatory understanding, and complete checkout securely.
Related Reading
Need Help Buying Retatrutide in the UK?
For educational comparisons and dosing information, explore our detailed guides before placing an order.
Buy Retatrutide from Veyvora
Free UK delivery | Batch documentation included